FUS (Fused in Sarcoma) is the causative protein and an important target for familial amyotrophic lateral sclerosis (ALS). Ryo Kitahara discovered two types of liquid-liquid phase separation (LLPS) by gradually changing the pressure applied to an aqueous solution of FUS, of which HP-LLPS (aberrant type) promotes abnormal aggregation of FUS. He found that low-molecular-weight substances such as arginine inhibit the formation of HP-LLPS and delay the process of abnormal aggregation.
Focusing on FUS, the protein responsible for familial ALS
Proteins are polypeptides composed of tens to hundreds of amino acids and are essential to life. Polypeptides generally adopt a specific three-dimensional structure to function as proteins in our bodies; however, when their structure is damaged for some reason, they form irreversible abnormal aggregates that cause various diseases. One such disease is ALS, where motor and respiratory functions are impaired due to the degeneration of motor neurons. Among the proteins associated with ALS, intercellular aggregation of the nuclear protein FUS has been elucidated as a cause of some familial ALS.
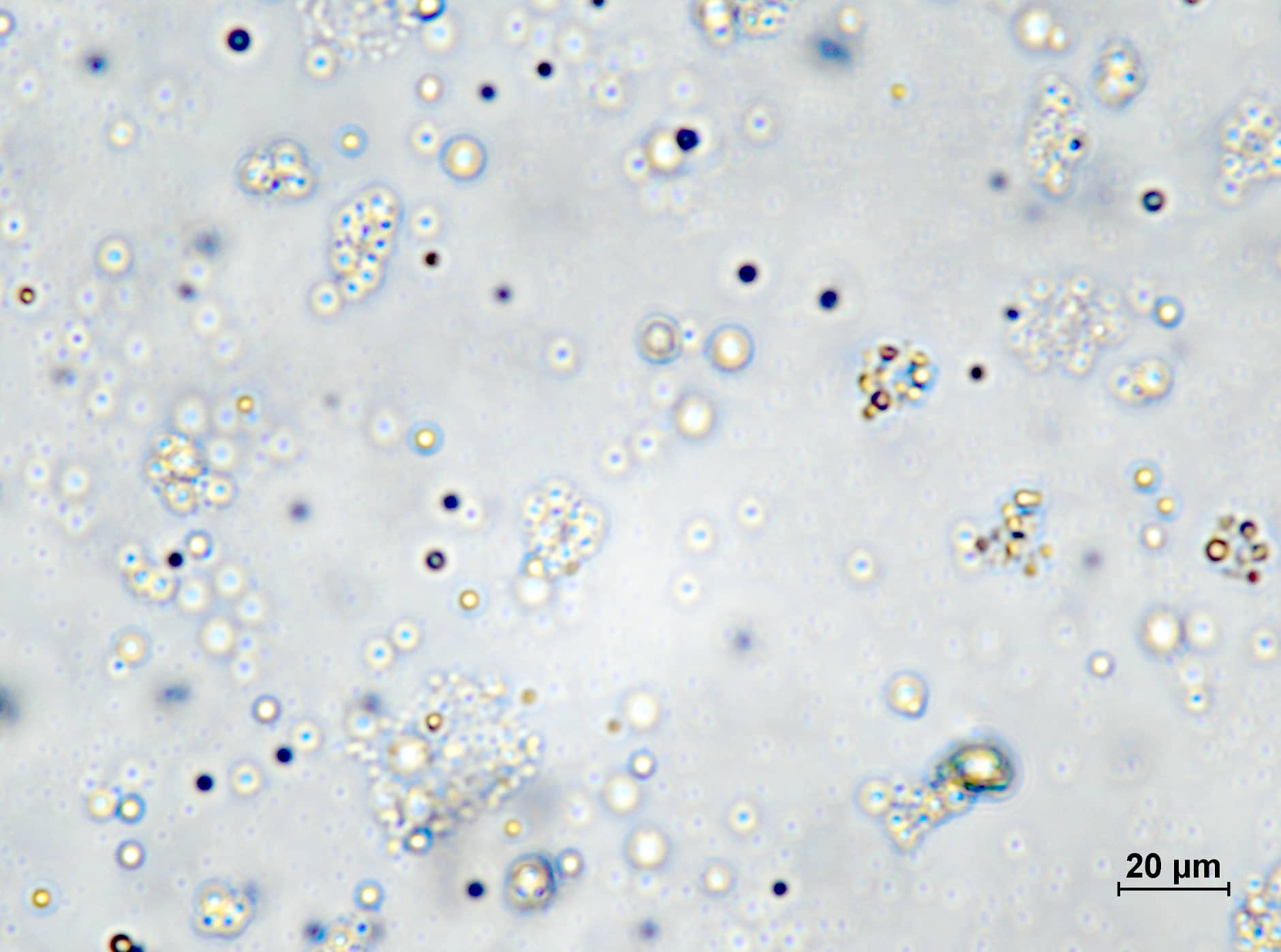
Several nuclear proteins, including FUS, form soft, droplet-like condensates inside the cell. The cell is filled with an aqueous solution of proteins and nucleic acids, whose concentration is not uniform. High-density solution droplets float around in the low-density solution. This phase-separated state is called LLPS.
Through their experiments in which high pressure was applied to aqueous solutions of FUS, a group of scientists led by Ryo Kitahara, Professor at the College of Pharmaceutical Sciences at Ritsumeikan University, found that FUS forms two types of liquid condensates: LP-LLPS, a low-pressure type that dominates at < 2,000 atm, and HP-LLPS, a high-pressure type that is abundant at pressures 2,000 atm or higher.
They observed that abnormal aggregation, which is the liquid-solid transition of FUS, was accelerated in HP-LLPS, while low-molecular-weight compounds such as arginine (an amino acid), suppressed the formation of HP-LLPS and delayed FUS aggregation. "LLPS in aqueous protein solutions is a relatively recent discovery. The number of publications on the protein LLPS has exploded since 2012. It is now assumed that cells are filled with LLPS of different proteins. By focusing on LLPS, we can now explain the causes of some previously unknown phenomena," explains Kitahara.
Searching for new LLPSs by changing the pressure applied to proteins
The physical state of matter is determined by pressure and temperature. When cooled at 1 atm, liquid water forms solid ice. But, according to Kitahara, at high pressures above 10,000 atm, ice forms even at room temperature. New LLPSs can be discovered by studying protein structures in environments with varying temperature and pressure. Experiments conducted at normal pressure may reveal only a small part of nature, a bright spot, so to speak.
The advantage of using pressure perturbations is that they not only reproduce the extreme environments of the earth, but also lead to the discovery of unknown reactions and phenomena that have not been observed at atmospheric conditions. When heating an object, the object closer to the heat source heats up faster, creating a temperature gradient, whereas hydrostatic pressure does not create a gradient because it is applied instantaneously and uniformly to the object. Using spectroscopic and microscope techniques with a combination of the pressure-jump, in which the pressure can be changed by more than 1,000 atm in a few seconds, Kitahara was able to observe the formation and disappearance of LLPSs in real time. He also found that the formation and disappearance rates of HP-LLPS are considerably slower than those of LP-LLPS.
Why could HP-LLPS, which is common at higher pressures, cause familial ALS in a normal pressure environment? Kitahara admits that they have not found any direct evidence for the presence of HP-LLPS at normal pressure. However, by gradually changing the pressure applied to aqueous solutions, he and his colleagues have confirmed that the two types of LLPS are in physical equilibrium. He believes that HP-LLPS exists at all pressures, although it is less common than LP-LLPS due to greater instability under atmospheric pressure.
"Proteins exist in a chemical equilibrium between at least two states: native and denatured states. This means that denatured proteins are present even in healthy cells. Under atmospheric conditions, the denatured state is extremely uncommon, making protein-protein interaction and aggregation unlikely. On the other hand, in people with familial diseases, denatured proteins are more abundant than usual, which increases the likelihood of interaction and aggregation," says Kitahara. Although LLPS is a physical and not a chemical equilibrium, the scenario is similar: the presence of HP-LLPS may be more likely in people with familial ALS. Another scenario suggested by Kitahara is the change in protein properties due to amino acid mutations. A single change in an amino acid sequence can drastically alter not only the stability of the protein, but also its aggregation property. The aberrant amino acid sequence may enhance the aggregation of HP-LLPS.
Validating the inhibitory effect of arginine on the formation of HP-LLPS with a view to drug discovery
Arginine is commonly marketed as a dietary supplement with claims of building muscle or strengthening the immune system. Is it possible to prevent ALS with arginine? Kitahara comments as follows: "The advantage of using arginine is that it acts selectively on HP-LLPS. Research on protein LLPS is still in its infancy, and it is possible that we discover a new role of LLPS that is essential for cells. The inhibition of all LLPS states including the LP- and HP-LLPS states might have unexpected side effects. Therefore, we first need to establish an approach to drug development by targeting HP-LLPS."
Unfortunately, we will not be able to claim that arginine can prevent or treat ALS any time soon. One of the challenges is the process of drug delivery into cells. With oral or intravenous administration, only a small fraction of amino acid concentration in the blood reaches the cells. Kitahara and his team have started investigating the effects of arginine on the formation of protein condensates in human cells. They are also searching for other chemical compounds that can suppress the formation of HP-LLPS in smaller amounts and have also begun to design new chemical compounds using computer-assisted techniques in collaborative research. "We hope to contribute to drug development by discovering new effects of existing chemical compounds and searching for novel drug candidates," Kitahara concludes.